Martin Kulke's paper on simulation based structure prediction of the fibronectin-interchain module is published in the Journal of Chemical Information and Modeling.
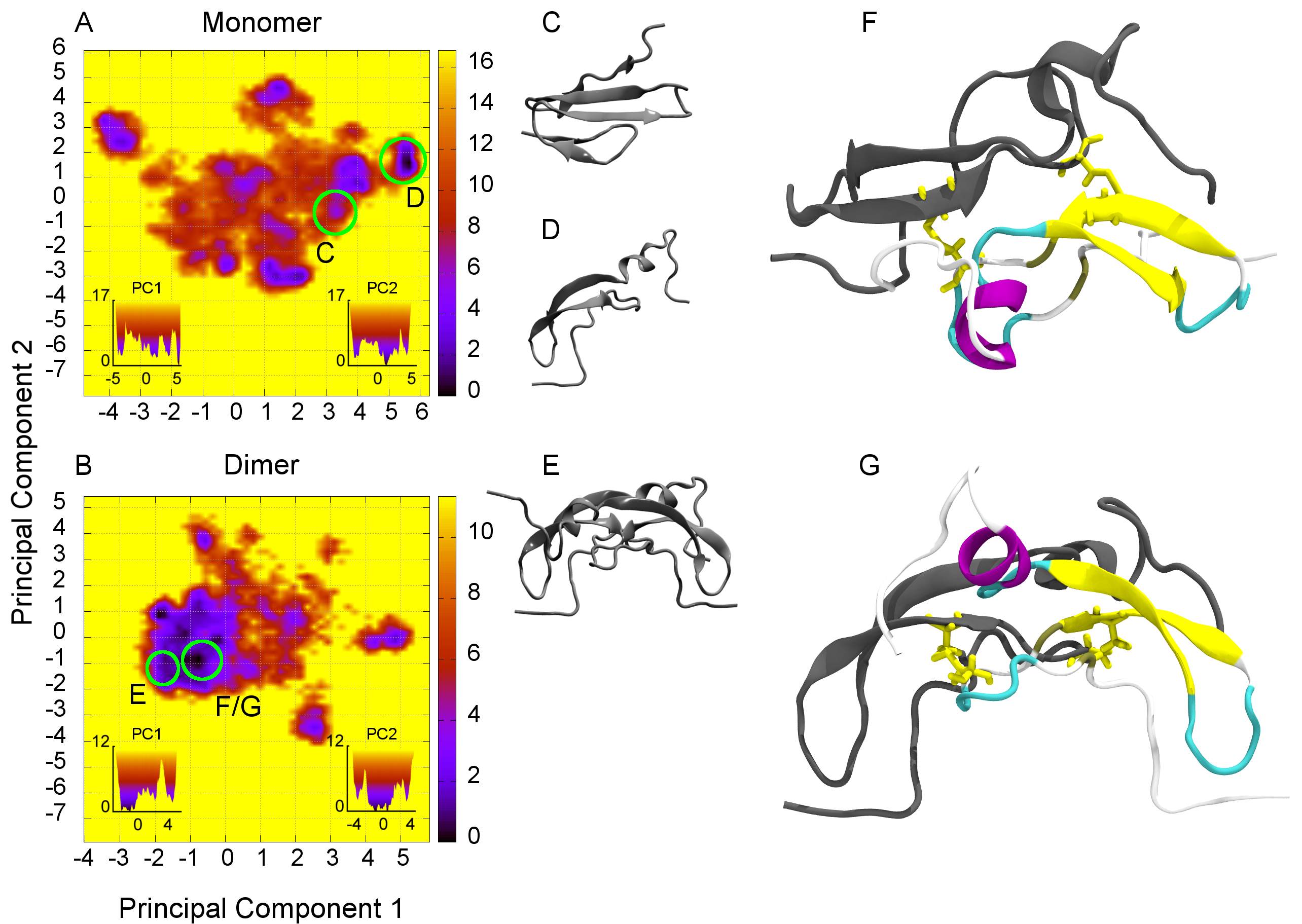
Fibronectin is a key player in cell adhesion and is involved in the complex mechanobiology of the ECM. Many parts of its sequence and its modules have been studied and understood, however the biological relevance of the dimerisation site at the C-term is still unclear. It is known that fibronectin is only phosphorylated in the CTXL domain, but no results have been presented to date, which indicate a biological function based on this phosphorylation. Utilizing our novel TIGER2hs simulation algorithm, the first ever structure for the Fibronectin Cross-Linking Module (CTXL) is found and its structural stability is heavily influenced by the phosphorylation state. This is indicating a biological relevance, previously unattended.