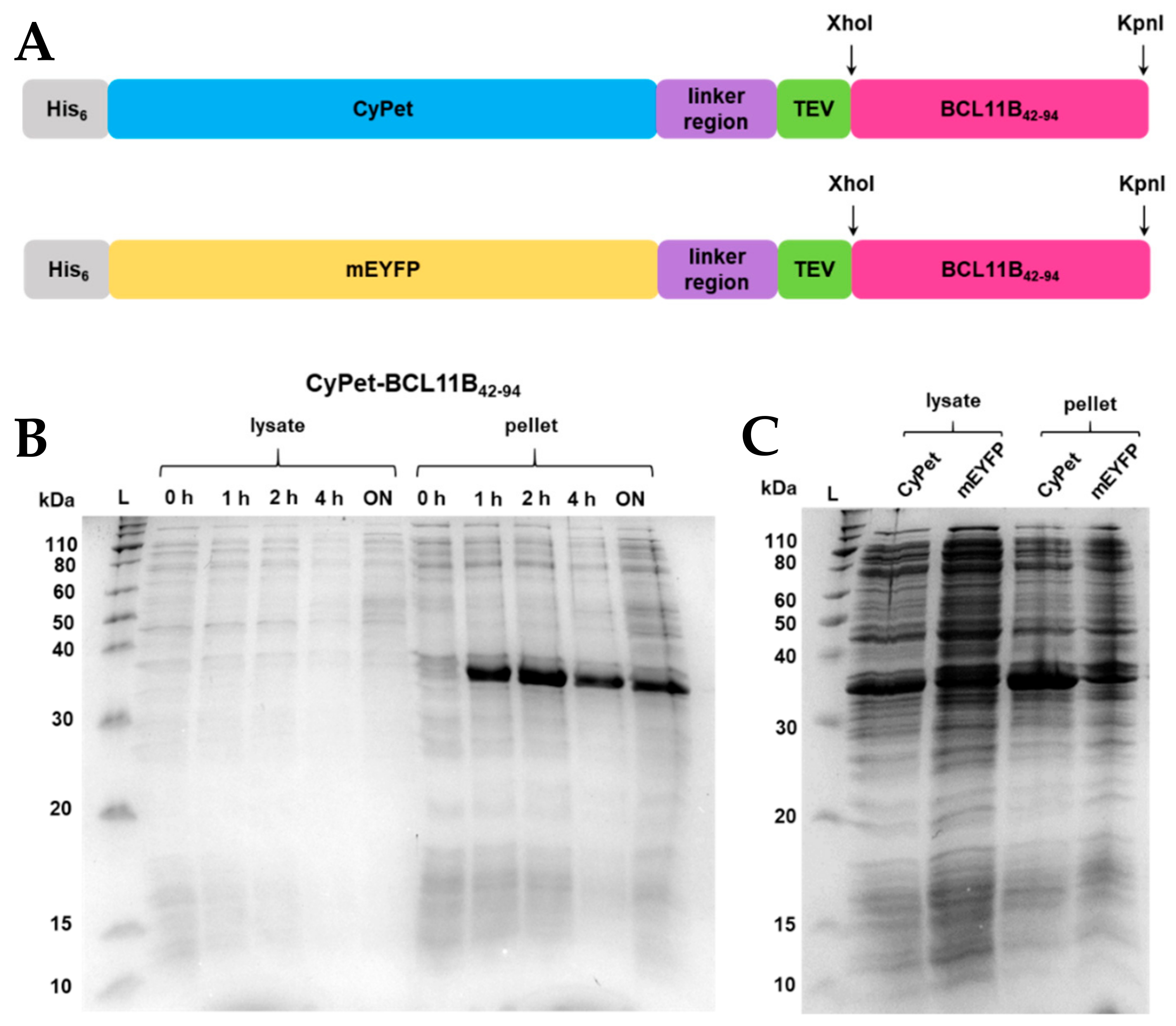
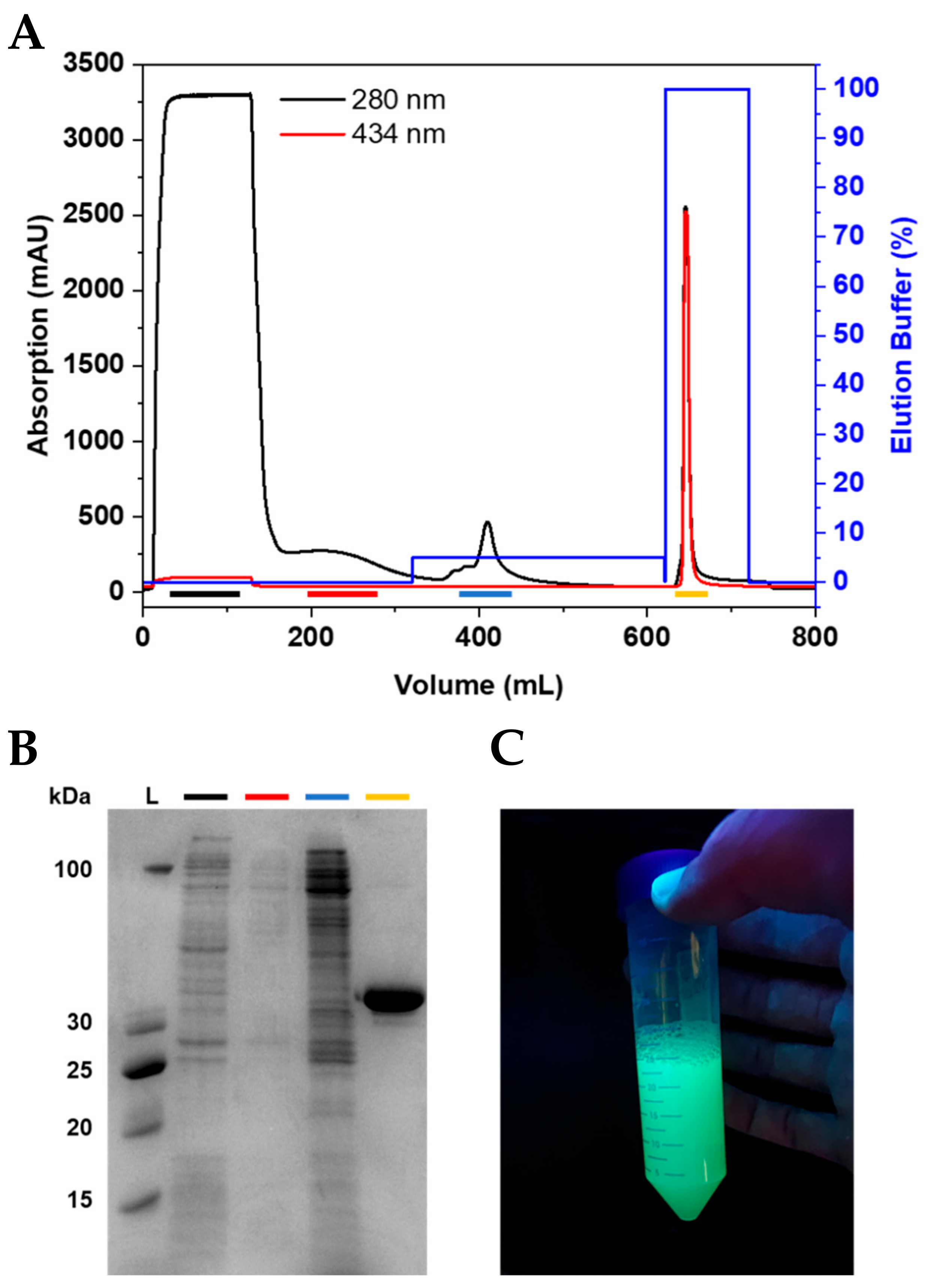
Anne's paper on Easy Expression and Purification of Fluorescent N-Terminal BCL11B CCHC Zinc Finger Domain is published in Molecules. Congratulations Anne!
Anne's paper on Easy Expression and Purification of Fluorescent N-Terminal BCL11B CCHC Zinc Finger Domain is published
Zurück zu allen Meldungen
