Felix's paper on Structural and Biophysical Insights into SPINK1 Bound to Human Cationic Trypsin is published in International Journal of Molecular Sciences. Congratulations Felix!
Felix's paper on Structural and Biophysical Insights into SPINK1 Bound to Human Cationic Trypsin is published
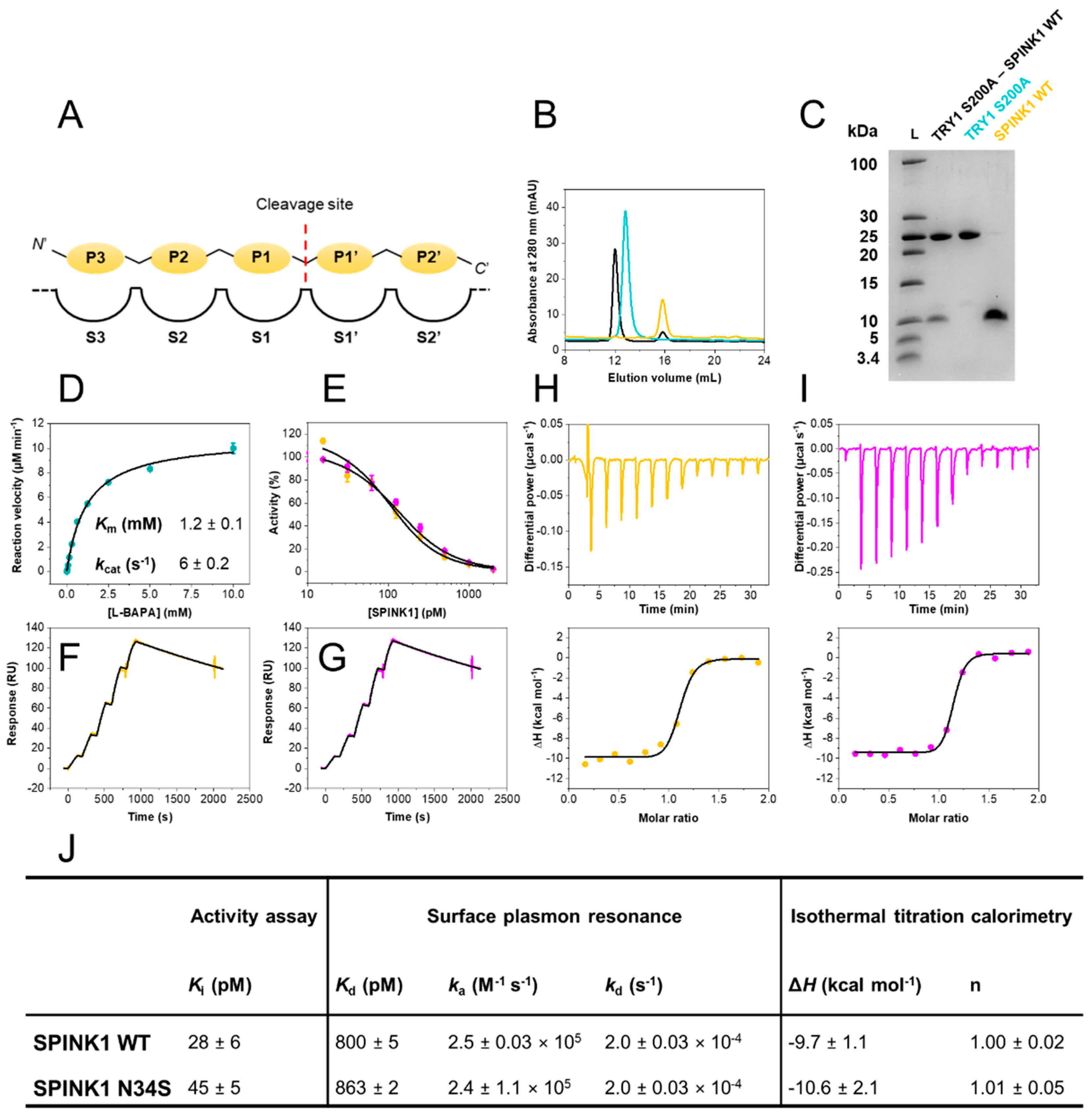
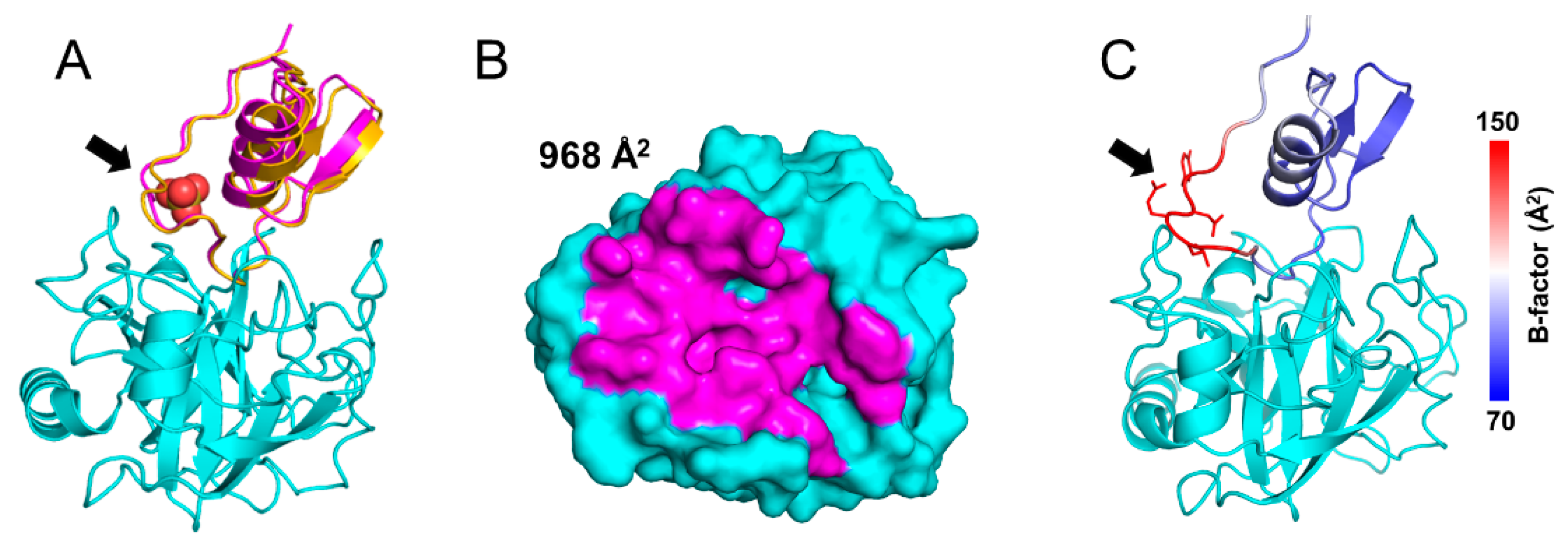
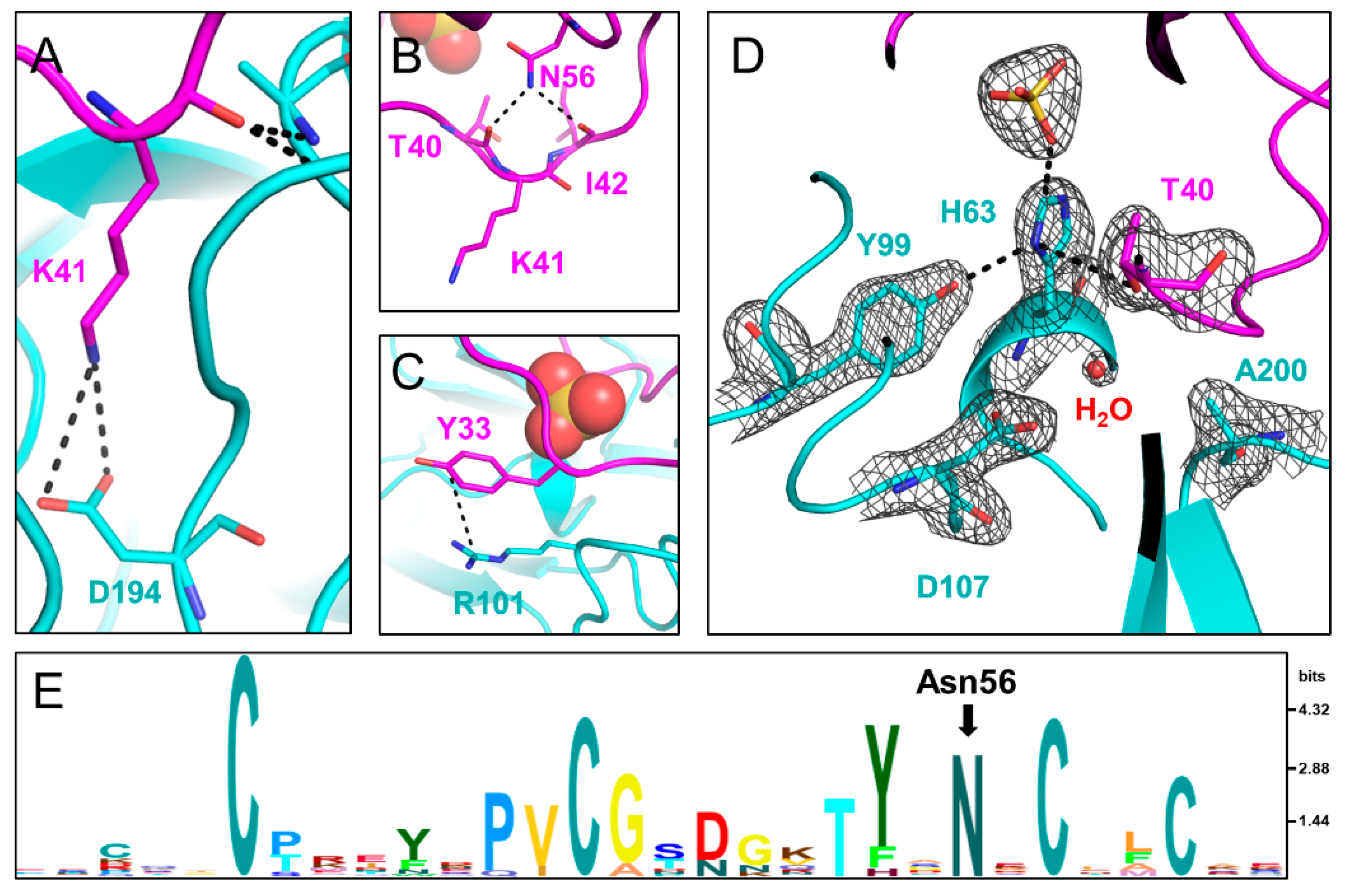
Zurück zu allen Meldungen
