Research
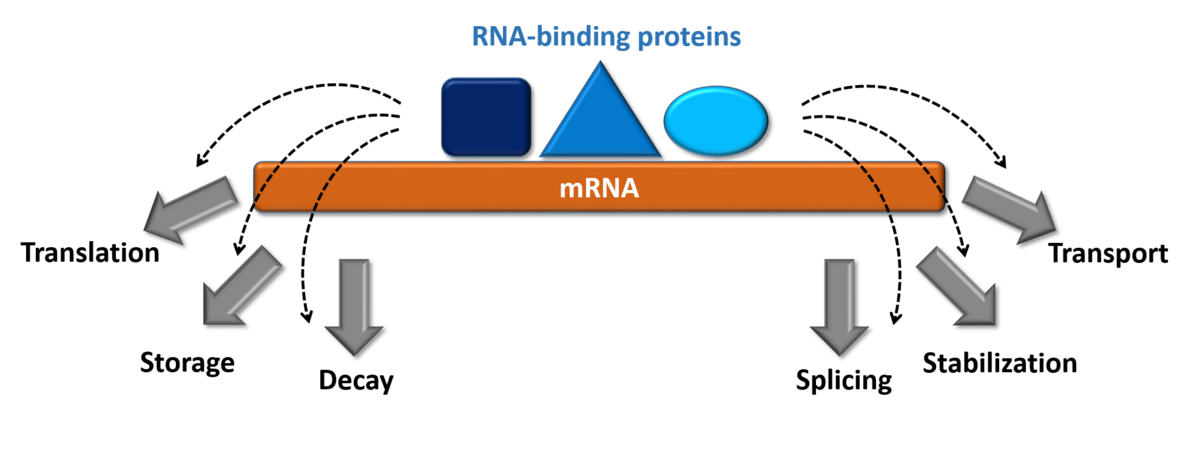
How do proteins achieve the necessary specificity in RNA-recognition during gene regulation?
Content to come...


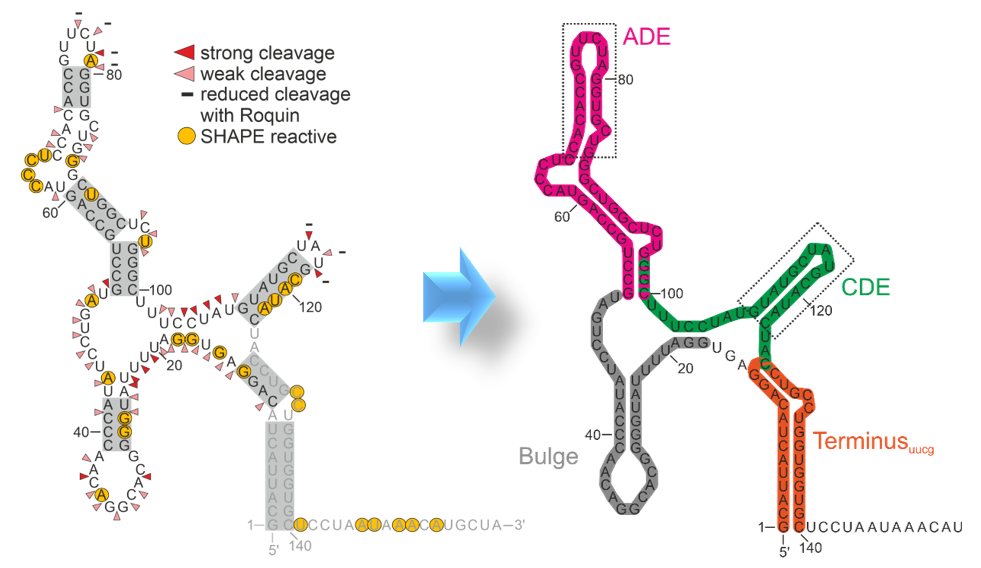
Specific RNA fold recognition. Content to come...
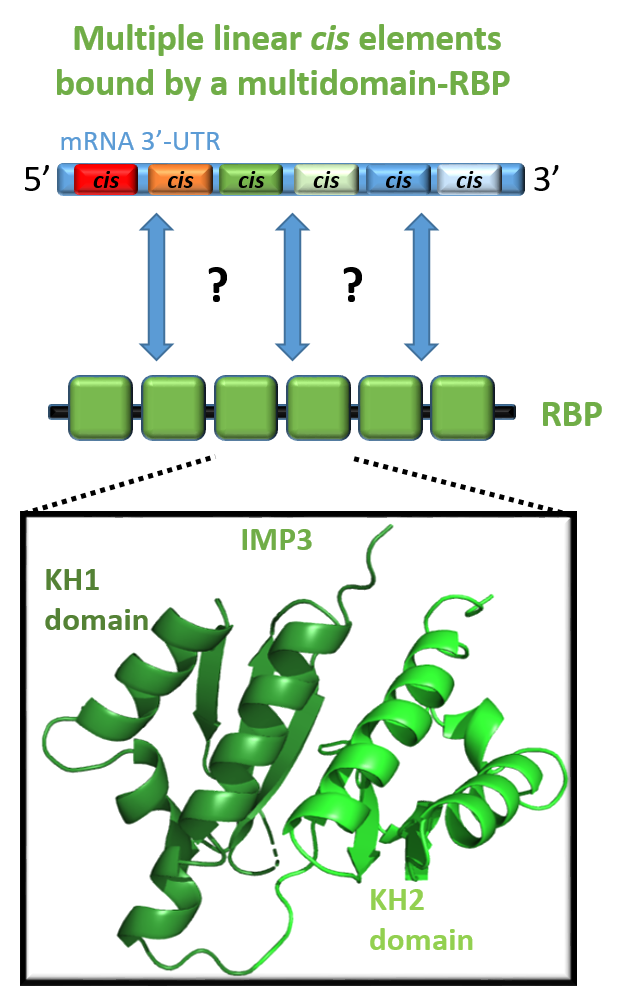
The specific recognition of linear RNAs by multi-domain RNA-binding proteins. Content to come...